
Irritation Tests
IRRITATION TESTS- ISO 10993-10,GB/T 16886.10,EN ISO 10993-10,USP Chapter<88>,GB/T 14233.2, YY/T 0268, YY/T 0127.13
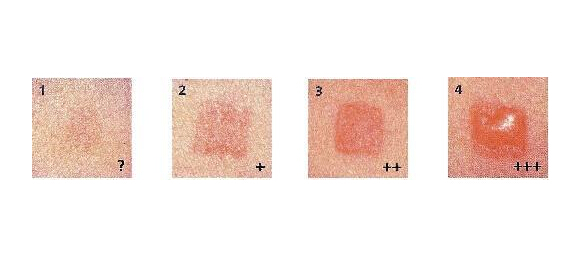



Introduction of Irritations Tests
Skin Irritation Test | Local reversible damage after skin contact with the test substance, skin irritation reaction generally does not involve the immune system, and its typical manifestations are erythema or edema. Skin irritation test: Quantitatively apply different doses of the test substance on the intact skin on one side of the depilated part of the experimental animal (rabbit is the first choice) and apply it to fix it, and use the other side as a self-reference to observe the absorption And evaluate the degree of irritation response. |
Intradermal Irritation Test | The medical device extract is injected into the rabbit skin to evaluate the potential irritation of the test product to the contact tissue under the test conditions. |
Ocular Irritation Test | Evaluate the potential of the material to produce eye irritation under the test conditions. Any material that has been shown to be skin or eye irritants, or pH ≤ 2.0 or ≥ 11.5 should not be tested again, and should be labeled as potential penile, rectal, and vaginal irritants. |
Oral Mucosa Irritation Test | Oral irritation tests should only consider materials that are expected to come into contact with oral tissues, and should only be considered when safety data cannot be obtained by other methods. Any material that has shown skin or eye irritation, or PH value ≤ 2 or PH ≥ 11.5 is not suitable for oral mucosal irritation test, and can be marked as potential oral tissue irritation. |
Vaginal, Penile, Rectal Irritation Test | Evaluate the potential of the material to produce vaginal, penile, and rectal irritation under test conditions. Only materials that are expected to come into contact with vaginal, penile, and rectal tissues should be considered, and should only be considered when safety data cannot be obtained by other methods. Any material that has been shown to be skin or eye irritants, or pH ≤ 2.0 or ≥ 11.5 should not be tested again, and should be labeled as potential vaginal, penis, and rectal irritants. |
STANDARDS:
ISO 10993-10,GB/T 16886.10,EN ISO 10993-10,USP Chapter<88>,GB/T 14233.2, YY/T 0268, YY/T 0127.13
Skin Irritation Test | The contact parts were recorded at 1h, 24h, 48h and 72h after the removal of the application. If there is a persistent injury, it may be necessary to extend the observation period to assess the reversibility or irreversibility of the injury, but the duration need not exceed 14 days.
Describe and score the erythema and edema reactions of the skin at each contact site at each specified time, according to the classification system prescribed by the standard, and record the results for the issuance of the test report. |
Intradermal Irritation Test | The condition of each injection site was observed and recorded immediately after injection at (24±2) h, (48±2) h and (72±2) h. Tissue response to erythema and edema at each injection site during each observation period was scored according to the corresponding scoring system, and the test results were recorded. At (72±2) h, an appropriate living dye, such as Trypan blue or Evans blue, could be injected intravenously to show that the stimulated area was helpful for response evaluation. |
Ocular Irritation Test | The eyes of each animal were examined at approximately (1±0.1) h, (24±2) h, (48±2) h and (72±2) h after the injection. If persistent injuries are present, the observation period should be extended to determine the progression and reversibility of the injuries, but the delay should be up to 21 days. |
Oral Mucosa Irritation Test | Cheek pouches were observed after sample removal and before each contact; Describe the general condition of cheek pouches and score the erythema reaction of cheek pouches in each observation period according to the scoring system given in the corresponding table. Cheek pouch was observed by naked eye (24±2) h after the last contact, and the hamsters were sacrificed painlessly. Representative samples of cheek pouches were collected and fixed with appropriate fixation solution for histological examination. |
Vaginal, Penile, Rectal Irritation Test | The experimental penis and foreskin, vagina and rectum were compared with the corresponding parts of control animals. The scores for each observation were added up and divided by the number of observations to give the average score for each animal. After 48 hours of observation, the animals were killed immediately, and the test sites were cut off and fixed with appropriate fixative for histological examination. The microscopic examination results of all animals in the experimental group were graded, and the total number was divided by the number of animals observed to obtain an average value of the group. The control group was calculated by the same method. The stimulus index was obtained by subtracting the mean score of the test group from the mean score of the control group. |
PERIOD
Skin Irritation Test | 1~2 weeks, please contact EPIN for details |
Intradermal Irritation Test | 1~2 weeks, please contact EPIN for details |
Ocular Irritation Test | 1~2 weeks, please contact EPIN for details |
Oral Mucosa Irritation Test | 8 weeks, please contact EPIN for details |
Vaginal, Penile, Rectal Irritation Test | 8 weeks, please contact EPIN for details |
SAMPLE REQUIREMENTS——SINGLE POLAR:
Ø ISO 10993-10,GB/T 16886.10,EN ISO 10993-10,GB/T 14233.2, YY/T 0268, YY/T 0127.13
Items | Status | Number | Samples (Total) | ||
Quality | Surface Area | Volume | |||
Skin Irritation Test | Solid | 1 pcs | 2 g | 60 cm2 | - |
Liquid | - | - | - | >10 mL | |
Intradermal Irritation Test | Solid | 1 pcs | 2 g | 60 cm2 | - |
Liquid | - | - | - | >10 mL | |
Ocular Irritation Test | Solid | 1 pcs | 2 g | 60 cm2 | - |
Liquid | - | - | - | >10 mL | |
Oral Mucosa Irritation Test | Solid | 1 pcs | 2 g | 60 cm2 | - |
Liquid | - | - | - | >10 mL | |
Vaginal, Penile, Rectal Irritation Test | Solid | 5 pcs | 10 g | 300 cm2 | - |
Liquid | - | - | - | >50 mL | |
Ø USP Chapter<88>
Item | Status | Number | Samples (Total) | |
Quality | Surface Area | |||
Intradermal Irritation Test | Solid | 4 pcs | 8 g | 240 cm2 |
Copyright © EPINTEK GROUP
Powerd by PEERHI
EPINTEK GROUP
Tel: +86 21 54736833
Address: 4th Floor, T2 Wanjin Center, Lane 360, Xinlong Road, Minhang District, Shanghai
E-mail: stefanie.sun@epintek.com




We Focus on the Demand for Innovation
