Core Business
Foster Collaboration with Innovative Solutions
We provide services such as base screening, scheme design, initiation training, data management, biostatistics, clinical monitoring, and third-party inspection for your medical devices (including IVDs).
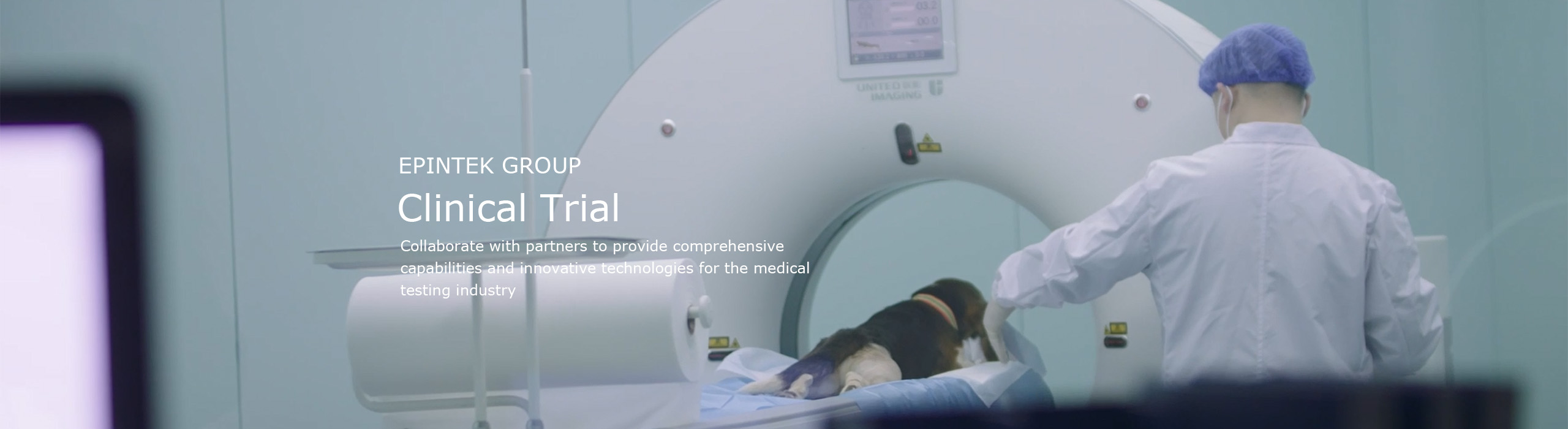
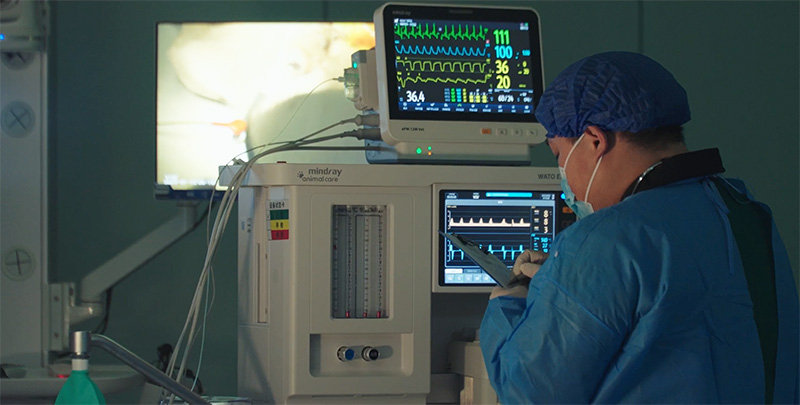
Provide preclinical animal experiments, biocompatibility, microbiology and other preclinical validation services
Collaborate with partners to provide comprehensive capabilities and
innovative technologies for the medical testing industry
Services and Solutions
Class II, III, and innovative medical devices that require clinical trials, including active, passive, implantable, IVD, and other devices.
We provide services such as consultation on the compilation of technical documents, consultation on registration and certification regulations, and training.
NEWS
Follow EPINTEK's Updates and Industry News
Provide comprehensive testing services for clients in the medical industry
EPINTEK Test Platform